Beaucouzé, 29 September 2020 - Ceva Santé Animale has increased three-fold its production capacity for bacterial autogenous vaccines (primarily, for pigs and poultry) at its French plant. The Ceva Biovac campus, near Angers, includes state of the art technology for the selection and characterization of bacterial strains in order to rapidly prepare the most effective custom vaccines The extensive experience gained in France in rationalising antimicrobial use through the use of autogenous vaccines will be rolled out through Ceva subsidiaries around the world.
A French centre for excellence with European reach
This €8 million innovative project confirms Ceva’s commitment to improving preventative animal health and reducing the use of antibiotics. The addition to the existing Ceva Biovac site will further reinforce Ceva as the leading provider of autogenous vaccines for animals.
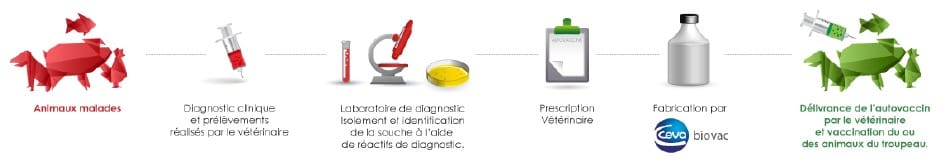
Autogenous vaccines are custom solutions for veterinarians and farmers, manufactured directly from disease strains collected from animals on a specific farm.
Ceva Biovac is equipped with the most up to date vaccine technologies, bringing optimal security and surpassing all European (BPF1) and international (GMP2) regulations. The tailored manufacturing offers flexibility in terms of batch sizes (1 to 200 L) and a production capacity of 300 batches per week.
Today, Ceva has 5 sites dedicated to producing autogenous vaccines around the world (Canada, France, Germany, USA and the United Kingdom), with the new Ceva Biovac facility become acting as a reference site in developing new processes and R&D, which will then be deployed internationally.
Autovaccines: custom treatments manufactured with short lead times
Vaccines, and especially auto vaccines, are the main alternative treatment to antibiotics in the fight against bacterial diseases. Based on a bacterial sample taken by the veterinarian from an animal suffering from an infectious pathology, Ceva Biovac isolates the pathogenic agents to produce a specific, custom-made batch of vaccines with a short turnaround of 5 weeks. The vaccines are then administered to the farm animals in question, under the supervision of a veterinarian.
Far from replacing traditional forms of vaccination, they offer a complementary model when there are no vaccines available with marketing authorisation.
Committed to better preventative health
“For over 10 years now Ceva has been firmly committed to better preventative health. Today, vaccines represent almost 50% of our portfolio of products. This strategy illustrates our efforts in the fight against antimicrobial resistance, which places veterinarians at the heart of the fight against microbial diseases. Along with vaccines, custom vaccines are an alternative to medication and a major tool for reducing the use of antibiotics in the livestock sector”, said Dr Marc Prikazsky, Chairman & CEO of Ceva Santé Animale.
Ceva Santé Animale in numbers
€1.2 billion in sales in 2019
10% of sales revenue reinvested in R&D
Commercial presence in 110 countries
12 R&D centres worldwide, including 4 in France
6,000+ employees, with more than 1,600 in France
Ceva Biovac in numbers
10 million euros in revenue generated by campus products
€8 million in investments made between 2016 and 2020
80 employees
Innovation capacity:
- 1,500 m² production laboratory including 2 sterile laboratories, 4 sterile antigen production workshops and a sterile distribution formulation workshop (capacity will eventually be multiplied by 3)
- 200 m² bacterial identification and reagent production laboratory that identifies 4,000 pathogenic strains per year and produces around 100 serological reagents
(1) BPF : Bonnes Pratiques de Fabrication
(2) Good Manufacturing Practices
Contact Ceva
Martin Mitchell - Directeur de la Communication Groupe - martin.mitchell@ceva.com

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam